Clinical data management gives you the tools to organize, monitor, and protect every piece of data in clinical research. You ensure that each patient’s information stays accurate, complete, and secure throughout the research process. High-quality data supports valid results and helps you maintain data integrity. When you follow strong data management practices, you protect patient safety and improve the reliability of clinical trials. Regulatory compliance becomes easier because you record, validate, and audit data at every step. This careful approach reduces legal risks, speeds up research, and increases trust in your results.
When you take part in clinical research, you rely on clinical data management to keep every piece of information organized and secure. Clinical data management covers all the steps needed to collect, process, and protect data during clinical research studies. You see this process in action from the moment a patient joins a study until the final results are ready for review.
A clinical data manager designs and validates databases that store research data. You use electronic tools to collect information from patients, medical devices, and lab tests. These tools help you reduce errors and keep the data accurate. You also create edit checks and validation rules to make sure the data stays consistent and reliable. Medical coding helps you standardize terms, so everyone understands the results.
You handle data cleaning by checking for mistakes, missing values, or unusual results. When you find problems, you work with the research team to fix them. You monitor data quality and keep detailed records for audits. At the end of the study, you lock the database to prevent changes and archive the data for future reference. As a certified clinical data manager, you follow strict guidelines to protect patient privacy and meet regulatory standards.
Tip: Good clinical data management uses the ALCOA-CCEA principles. This means you keep data attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available.
You need strong clinical data management to make sure clinical research produces trustworthy results. Accurate and reliable data supports safe and effective treatments. When you manage data well, you protect patient safety by tracking adverse events and responding quickly to risks. You also help the research team make better decisions because they can trust the information they see.
Clinical data management plays a key role in meeting regulatory requirements. Agencies like the FDA and EMA expect you to keep data complete, accurate, and traceable. You create audit trails and follow data privacy laws, which helps you avoid legal problems and delays. A certified clinical data manager ensures that every step meets these high standards.
If you skip steps or make mistakes in data management, you risk delays and errors in clinical trials. Over 80% of trials face setbacks because of poor data handling. Only a small number of early-stage trials move forward to approval. When you use strong data management practices, you increase the chances of success for your research.
You also improve patient safety. Well-managed data lets you spot patterns in adverse events and act quickly. You use advanced tools, like AI and machine learning, to analyze data and support clinical decisions. This approach leads to better care and outcomes for patients.
Here are some key reasons why clinical data management matters in clinical research:
A certified clinical data manager brings expertise to every stage of the process. You help your team stay organized, meet deadlines, and deliver high-quality results. With strong clinical data management, you give clinical research the foundation it needs to succeed.
You start the clinical data management process by planning how you will collect data. You design clear case report forms (CRFs) that match your study’s goals. These forms can be paper-based or electronic. Today, most teams use electronic data capture (EDC) systems because they improve data quality and speed. EDC systems let you enter data directly, use built-in validation checks, and reduce errors. You can also collect data from electronic health records, lab reports, and patient-reported outcomes. This approach helps you gather accurate and complete data from many sources. However, you must watch for data silos and make sure all systems work together.
Tip: Using EDC systems can cut data entry time, lower costs, and boost efficiency compared to paper-based methods.
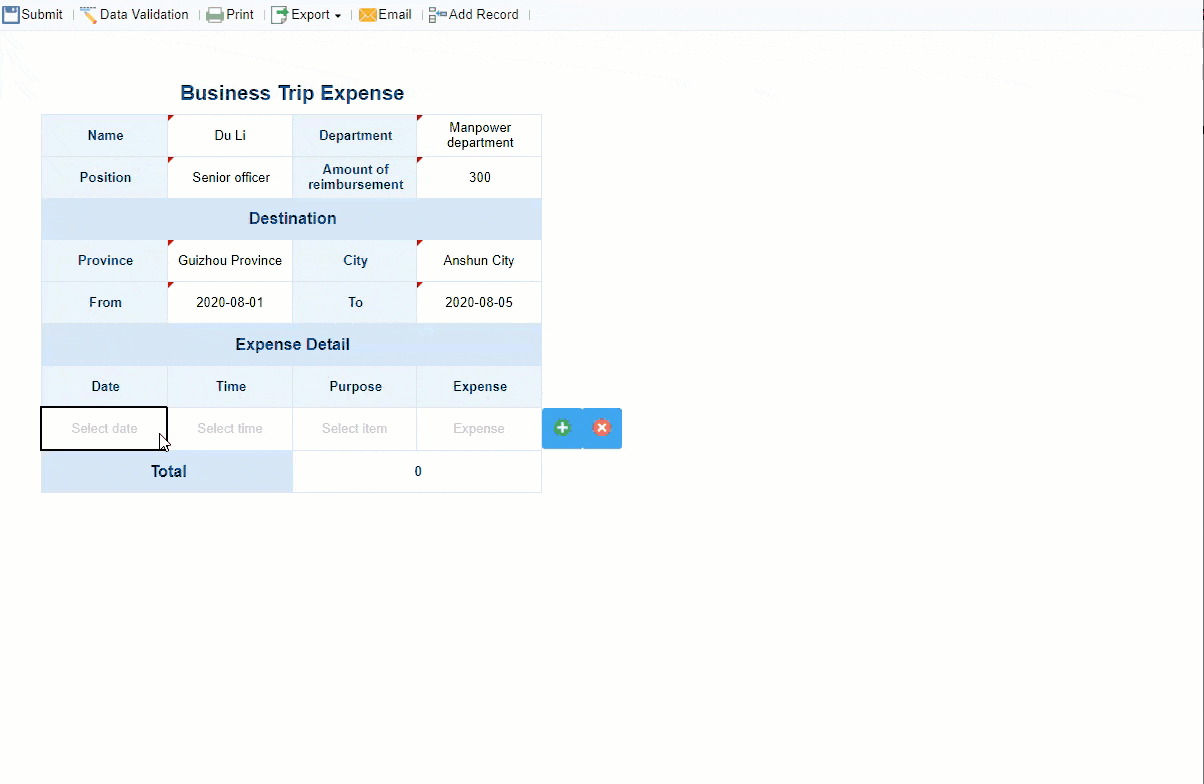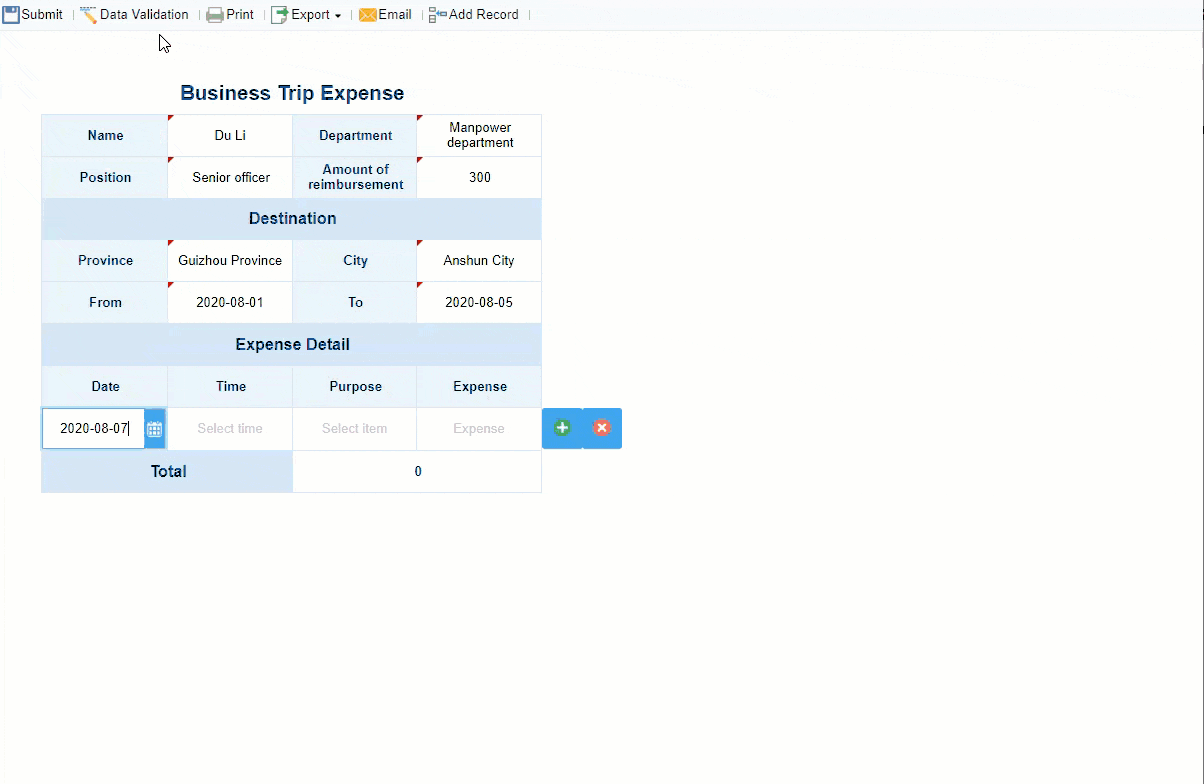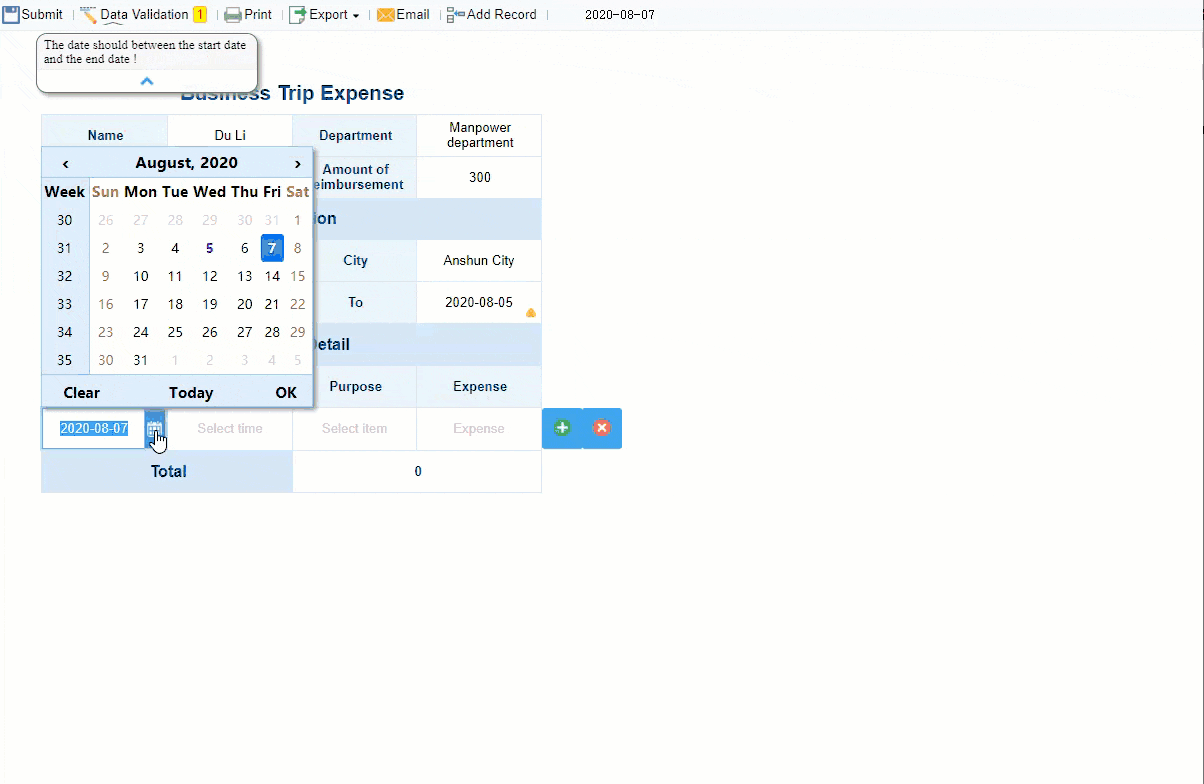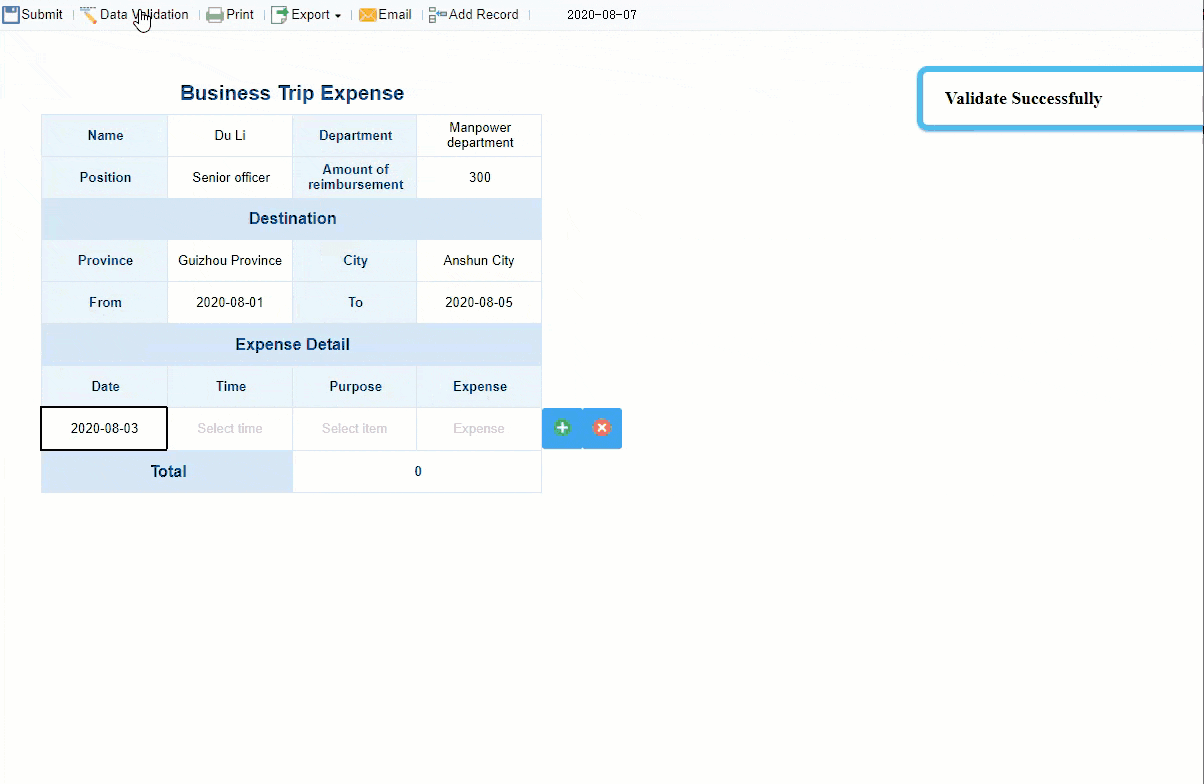
After you collect data, you need to check its quality. Validation is a key step in clinical data management. You use automated edit checks in your EDC system to spot missing values, out-of-range numbers, or logical errors. Hard edit checks stop you from moving forward until you fix the problem. Soft edit checks let you continue but require you to resolve the issue later. You also compare data with source documents to make sure everything matches. Validation includes reviewing, verifying, and cleaning data to remove errors and inconsistencies. This process supports data quality and helps you meet regulatory standards. If you skip validation, you risk poor data quality, delays, and even threats to patient safety.
When you finish data collection and validation, you move to the database lock step. You resolve all queries, clean the data, and get sign-off from key team members like the clinical data manager and principal investigator. You then lock the database so no one can make changes. This step protects data integrity and ensures your results are reliable. After the lock, you use the data for analysis and regulatory submissions. The database lock process is critical for quality and compliance, making sure your trial results stand up to review.
You see rapid changes in clinical trial data management because of new technology. FanRuan and its FineBI platform give you powerful tools to handle the growing complexity of clinical trial data. FineBI lets you connect to many data sources, including electronic health records, lab systems, and patient-reported outcomes. You can bring all your clinical trial data together in one place, making it easier to manage and analyze.
FineBI supports real-time data integration and ETL/ELT processes. You can automate the flow of clinical trial data from collection to analysis and reporting. This reduces manual work and helps you avoid errors. FineBI’s visual dashboards let you track trial progress, monitor patient safety, and spot issues early. You get a clear view of your data, which helps you make better decisions.
Security and compliance are top priorities in clinical data management systems. FineBI uses encryption for data at rest and in transit. You control who can access sensitive clinical trial data with role-based permissions and audit trails. These features help you meet strict regulations like GDPR and HIPAA. You can trust that your data stays safe and compliant.
Note: FanRuan’s solutions also support AI and machine learning. These tools help you find patterns in clinical trial data, predict risks, and improve patient outcomes.
You need strong data integration to get the most value from your clinical trial data. FineBI lets you combine data from different sources and standardize formats. This reduces errors and duplicate entries. You can track and report on clinical trial data in real time, even across multiple locations.

Here are some ways data integration and analytics improve your clinical trial data management:
| Benefit Area | Explanation |
|---|---|
| Real-time Data Collection | You monitor clinical trial data as it comes in, so you can act fast and avoid delays. |
| Enhanced Trial Efficiency | You spot and fix problems quickly, which keeps your trial on track. |
| Improved Data Quality | You catch errors right away, so your clinical trial data stays accurate and reliable. |
| Risk Management | You see protocol deviations or safety issues early, so you can respond before they escalate. |
| Regulatory Compliance | You keep up with standards like FDA and ICH through ongoing oversight and clear records. |
| Faster Time-to-Market | You use predictive analytics to speed up your clinical trial data analysis and reporting. |
With FineBI, you can handle large volumes of clinical trial data, keep your data secure, and support every step from collection to analysis and reporting. This technology gives you the confidence to run efficient, compliant, and successful clinical trials.
You see real progress in clinical research when you use advanced data management practices. Integrated real-world data and synthetic control arms help you design better clinical trials. These methods give you access to regulatory-grade historical data, which increases the chance of trial success. You can improve trial efficiency and meet regulatory standards more easily.
Electronic clinical outcome assessment (eCOA) tools let you collect higher-quality patient-reported data. You reduce errors compared to paper-based methods. This shift boosts patient engagement and makes your research more reliable. Risk-based quality management and targeted source data verification help you focus on the most important information. You increase data accuracy and use your resources wisely.
Structured content and data management (SCDM) speeds up drug development. You store and organize research data in a cloud-based library. This setup lets you reuse data for multiple regulatory submissions. You can update clinical trial applications quickly, which reduces delays and helps you respond to changes in your research. Automation and real-time analytics also cut down on manual work and speed up decision-making.
Tip: When you use advanced data management, you improve data quality, reduce errors, and help bring new treatments to patients faster.
You stand at the edge of exciting changes in clinical data management. Artificial intelligence (AI) and machine learning now automate many tasks, such as data review and anomaly detection. These tools help you find patterns in large datasets and predict trends in clinical research. Natural language processing lets you extract useful information from electronic health records, making patient data more accessible.
You can use AI-powered platforms to match patients to clinical trials more efficiently. Wearable devices give you real-time health data, which improves patient monitoring and safety. Blockchain technology increases data security and traceability, so you can trust your research records. Automated data extraction tools reduce manual errors and improve the quality of your research data.
AI and machine learning do not replace your expertise. Instead, they work with you to make clinical research more efficient and patient-centered. You still play a key role in interpreting results and making ethical decisions. As these technologies grow, you will see even more improvements in data quality, trial efficiency, and patient outcomes.
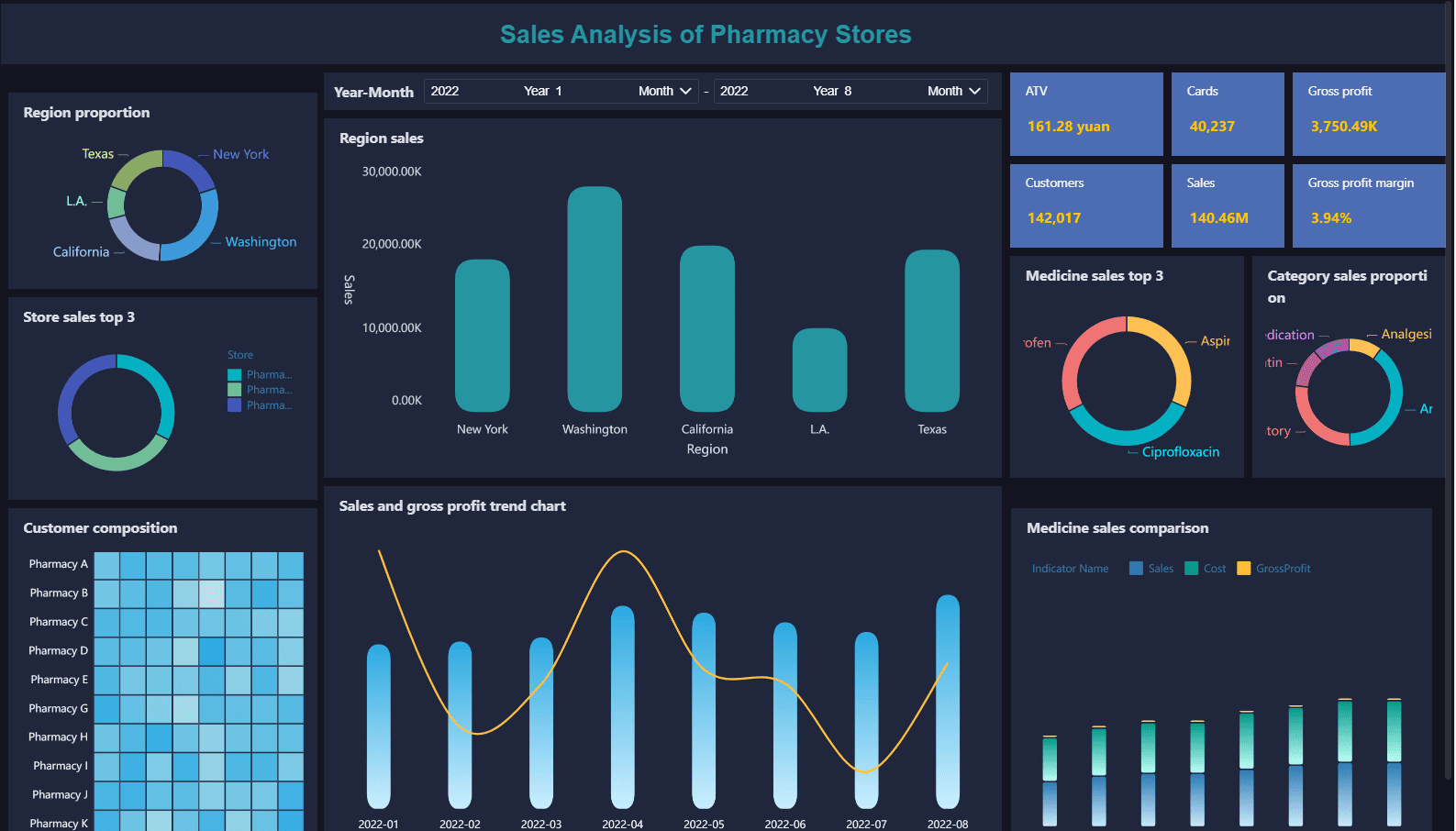
You can use clinical data management to transform how you run a pharmacy store. When you collect and analyze sales data, you spot trends in medication use and customer demand. This helps you keep the right products in stock and avoid waste. You also use data to track expiration dates and reduce losses from expired inventory.
Here are some ways you benefit from strong data management in pharmacy sales analysis:
| Aspect of Clinical Data Management in Pharmacy Sales Analysis | Description |
|---|---|
| Inventory Optimization | Analyze product usage and ordering patterns to keep inventory at the right level. |
| Demand Forecasting | Use past sales data to predict what customers will need next. |
| Waste Reduction | Track usage and expiration to cut down on unused or expired products. |
| Cost Reduction | Find ways to save money by understanding your purchasing habits. |
| Workflow Improvement | Streamline inventory and prescription processes to free up staff time. |
| Business Outcome Enhancement | Make decisions that boost revenue and customer satisfaction. |
When you apply these methods, you improve your pharmacy’s performance and meet regulatory standards. Real-world examples, like Walgreens’ renewal system, show how data-driven decisions lead to better business results.
You can also use clinical data management to analyze disease types and costs in hospitals. Electronic health records help you collect and organize patient data, but you need analytics to make sense of it. Predictive analytics lets you spot disease risks and plan treatments. You can also use prescriptive analytics to recommend actions that lower costs and use resources wisely.
When you use these data management techniques, you help hospitals deliver better care, control costs, and improve patient outcomes. You also support hospital administrators in making informed decisions every day.
You play a vital role in clinical research by using clinical data management to ensure patient safety and regulatory compliance. When you use advanced tools like FanRuan and FineBI, you improve data accuracy, speed up research, and support better patient outcomes.
Looking ahead, you will see cloud-based platforms, AI, and wearable technology transform how you manage patient data.
Click the banner below to try FineBI for free and empower your enterprise to transform data into productivity!
How to Start Your Career in Clinical Data Management Jobs
What is Healthcare Data Management and Why It Matters

The Author
Lewis
Senior Data Analyst at FanRuan
Related Articles
What is a data management platform in 2025
A data management platform in 2025 centralizes, organizes, and activates business data, enabling smarter decisions and real-time insights across industries.
Howard
Dec 22, 2025

Top 10 Database Management Tools for 2025
See the top 10 database management tools for 2025, comparing features, security, and scalability to help you choose the right solution for your business.
Howard
Dec 17, 2025

Best Data Lake Vendors For Enterprise Needs
Compare top data lake vendors for enterprise needs. See which platforms offer the best scalability, integration, and security for your business.
Howard
Dec 07, 2025